Gases On The Periodic Table
Noble gases were not discovered when mendeleev formulated his periodic table in 1869.

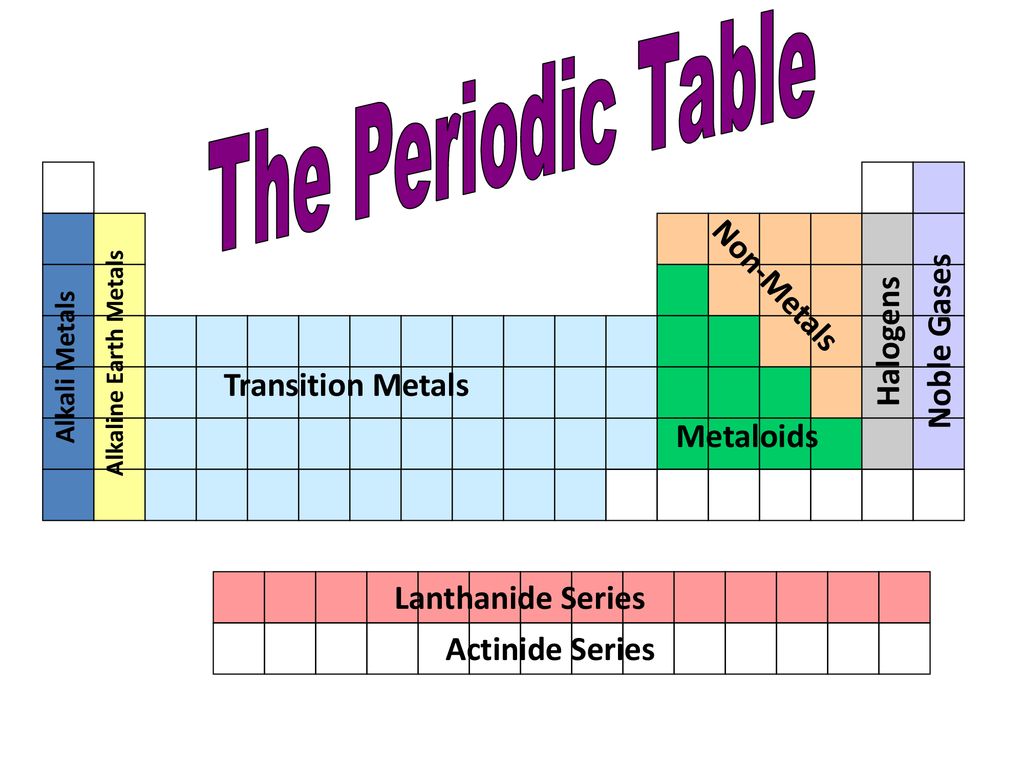
Gases on the periodic table. Each of the 13 elements has their own unique physical and chemical properties. Periodic table of the elements groups alkali metals alkaline earth metals blocks gases stp halogens lanthanidesactinides liquids stp main group metalloids metals noble gases non metals solids stp transition metals. Argon and helium can not be. For the first six periods of the periodic table the noble gases are exactly the members of group 18.
Periodic table was first invented by dimitri mendeleev from russia. Both these terms are now known not to be strictly correct. The inertness of noble gases makes them. When the elements are arranged in order of increasing atomic number there is a periodic pattern in their physical and chemical properties.
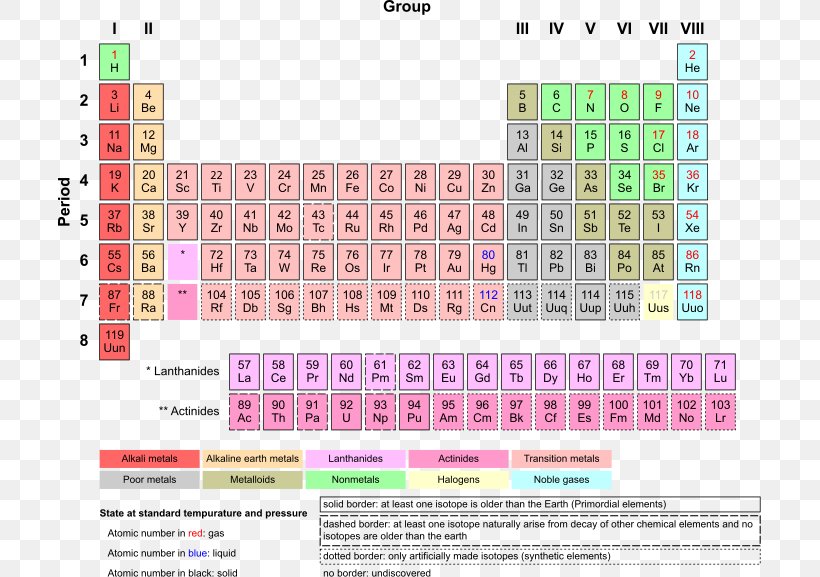
Periodic law states that. There are 110 elements in the new periodic table. Periodic table or periodic chart of elements showing gases. Helium he neon ne argon ar krypton kr xenon xe radon ra and element 118 uuo occupy the rightmost group of the periodic table.
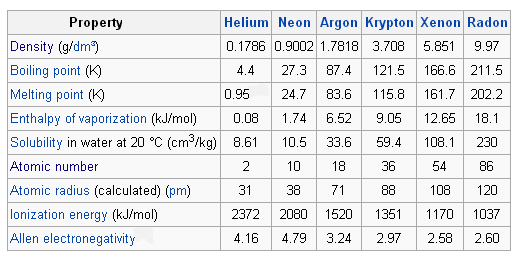
Some noble gases have been shown capable of forming compounds mainly with fluorine. Radon helium xenon neon krypton and argon are eight noble gases. However from the chemical point of view glass is the only material that could represent almost all elements of the periodic table inside itself showing the effect of the periodic law on properties of the final material. Its chemistry has not yet been investigated.
Noble gases are typically highly unreactive except when under particular extreme conditions. Noble gases were also known as inert gases and rare gases. Oganesson og is variously predicted to be a noble gas as well or to break the trend due to relativistic effects. In this paper we show the most remarkable examples demonstrating that.
Mercury hg and bromine br are the only elements in the periodic table that are liquids at room temperature. He had arranged the elements by increasing atomic masses.